US Free Classifieds
Most Popular Online Classifieds in USA. No Sign up, No Email Required to Post.
Canada Free Ads
Free Online Classifieds in Canada.
UK Free Ads
United Kingdom Free Ads Website.
100% Free Ad Posting.Canadian Pharmacy Meds is one of Canadas largest and most trusted certified mail-order pharmacies. Serving over 250,000 patients worldwide, we strive to provide our patients with quality brand and generic medications at savings of up to 90%. At Canadian Pharmacy Meds, quality and service are our top priorities. We are an approved member of the Canadian International Pharmacy Association (CIPA , the International Pharmacy Association of British Columbia, and PharmacyChecker.com. These industry accreditations mean that our business practices and our licensed pharmacies are continuously monitored and regulated to ensure safety, quality, and reliability.
Since our establishment, Canadian Pharmacy Meds has worked hard to earn your trust. We have customers from coast to coast and continue to grow every year. When filling your prescriptions at Canadian Pharmacy Meds, you are guaranteed to receive safe, effective, and affordable medications shipped to you in the manufacturers original packaging. All of our packages are also fully insured against loss or damage.
CanadianPharmacyMeds.com
PO Box 97176
Richmond Main Post Office
Richmond, BC
Canada V6Y 4H4
http://www.canadianpharmacymeds.com/
GlaxoSmithKline
(NYSE:GSK has agreed to market some of its over-the-counter brands in Europe to Omega Pharma.Belgium-based Omega Pharma is paying $614 million for brands such as stomach antacid Zantac and Nytol cold and allergy medicine .
Part of the deal includes transferring GSK’s Herrenberg, Germany factory to Omega Pharma.
Biotecnika Info Labs Pvt Ltd, Bangalore is a Parent Company which runs India’s Largest Biosciences Career & education Portal. Biotecnika is where in technology meets life sciences.
“We are a fast growing Bio-techno-media company with extremely talented and fun Loving people you would love to work with. Biotecnika is run by a group of Doctorate & post Doctorates of lifesciences & together we help in the upliftment of postgraduates from any lifesciences background. If you do not like processes, hate pyramid type organizations; love creativity, innovation and fun at work; you will love it here. You will find a very flexible work environment, which treats people like human beings”
- Post: Business Development Executive
- Company Name: Biotecnika Info Labs Pvt Ltd
- Location: in Bengaluru/Bangalore
- Experience: 0 to 3 yrs
- Salary: INR 1,75,000 - 2,50,000 P.A
- Opening(s : 5
Qualification & Duties
- Must be a MSc graduate in Bioscience
- 0 - 3 years Sales experience.
- MBA preferred
- Good written and oral communication
- Client follow ups
- Candidate should be willing to sell services and product.
- Candidates have to generate leads, qualify the leads on the sales strategies, identify the potential client, client interaction and maintain client relationship.
- Understanding the requirement of client and offering them with the appropriate services and product.
- Follow up with clients.
- Ability to meet multiple objectives within an organization.
- Work on Online marketing, Social Media Marketing, Email marketing, Tele Marketing as well as Field marketing
- Selling should be your signature statement.
- Maintaining relationship with clients and providing business leads, client interaction, team building, follow up with clients.
- Identify potential clients; manage existing account/customer relationships.
- Understanding the requirements of the clients & positioning an appropriate Solution
- Should be able to travel on demand as per the Company Policy.
- Liaisoning with Colleges / institutes for upliftment of biosciences
Desired Candidate Profile
Education:(UG - Any Graduate - Any Specialization, B.Com, B.B.A, B.Pharma, B.Sc,
B.Tech
/B.E. AND (PG - MBA/PGDM - Advertising/Mass Communication, International Business, Marketing, Other Management,
M.Tech
- Bio-Chemistry/Bio-Technology, Biomedical,
M.Sc
- Bio-Chemistry
- A minimum of 0 - 3 years of marketing experience
- Good organizational skills and the ability to understand detailed information.
- Basic IT and numeric skills.
- Good interpersonal skills to form effective working relationships with people at all levels.
- A proven track record of 'making a difference'.
- Creative, resourceful, detail-oriented, highly organized.
- Excellent verbal and written communication skills.
- Good Presentation skills.
- Time Management Skills.
- The potential to handle a leadership role.
- Candidate should have pleasing personality with excellent communication skills (Verbal & Written .
- Only candidates who can relocate to bangalore may apply
Executive Name:Sylvia Fernandes
Contact Company: Biotecnika
Address: #2628, 4th Floor 27th Main, Sector 1 HSR Layout, Landmark - CPWD Quarters BANGALORE,Karnataka,India 560102
Apply to Email Address :
jobs@biotecnika.org
Telephone: 80-91-65705331
Reference Id: MAR-10-BDE
Gland Pharma Ltd. is a leading Pharmaceutical Company approved by USFDA, involved in Manufacturing and marketing of Small Volume Parenterals, Pre-filled syringes and other Pharmaceutical Products
Post : QA Chemist
Experience : 2 to 5 yrs
Job Desciption :
a. Should have basic knowledge in sterile injecatble manufacturing.
b. Should be aware of current GMP(CRF/TGA/MCCMHRA requirements.
c. Good communication and dosumentation skills.
Education : M.Pharma,
M.Sc
- Bio-Chemistry,
Biotechnology
,
Microbiology
Desirable : Preference will be given to Sterile Injectable working area expereinced candidates.
Location : Hyderabad
Contact Details :
Contact Company : Gland Pharma Ltd
Location : Hyderabad
Email Address : hr@glandpharma.com
Deadline : 11.04.12
One Pennsylvania doctor in 2008 wrote 1,913 prescriptions for the antipsychotic drug Risperdal - a bit more than 5.2 per day in that leap year, counting weekends and holidays - costing Medicaid $341,273.71.
The top 10 prescribers in Pennsylvania's system that year wrote 9,557 Risperdal scripts costing Medicaid $1.76 million, according to figures provided by a state official to U.S. Sen. Charles Grassley (R., Iowa , who has pushed for disclosure of such information and the relationship between doctors and pharmaceutical companies.
The numbers raised questions for Grassley, and Pennsylvania officials sent letters to scores of doctors emphasizing the need for safety in prescribing antipsychotic drugs. Twelve were suspended, dropped from Medicaid, or are under investigation, according to a copy of a letter to Grassley released Tuesday by the state welfare department.
The numbers also play a role in the U.S. Department of Justice's efforts to fight health care fraud. In the case of Risperdal, the Justice Department is negotiating with Johnson & Johnson, whose Janssen subsidiary makes the drug, to address allegations that the company illegally promoted it to doctors and through Medicaid programs.
Medicaid is the taxpayer-funded insurance plan for poor Americans and is administered by the federal and state governments.
J&J previously disclosed that it set aside money to settle criminal and civil charges in the Risperdal litigation, though it had not specified the amount.
Reports over the weekend from the Wall Street Journal and Bloomberg News said the Justice Department had demanded a payment of about $1.8 billion, an increase from the $1 billion figure reportedly negotiated by the U.S. Attorney's Office in Philadelphia in December.
Spokesmen for J&J, the Justice Department, and the U.S. Attorney's Office declined to comment.
The $1.8 billion figure would be the largest settlement for a case involving a single drug, but some of the other big settlements also involved antipsychotic drugs.
"Both Sen. Grassley and the Department of Justice are making great headway in the battle against Medicaid fraud," said Allen Jones, the former investigator for Pennsylvania's Office of Inspector General whose findings were ignored by state officials in 2004.
Jones was fired by state officials when he took the information to the New York Times, but his whistle-blower lawsuit resulted in J&J's paying $158 million to settle charges that it illegally marketed Risperdal through the Texas Medicaid system. Jones will get a portion of that settlement. He now works as an adviser to attorneys in related litigation.
Eli Lilly & Co. paid $1.7 billion to settle charges of illegal marketing of its antipsychotic drug Zyprexa. Pfizer Inc. paid $2.3 billion to settle charges of illegal marketing of several drugs, notably Bextra, but also its antipsychotic Geodon. Late in 2011, GlaxoSmithKline P.L.C. said it had reached a deal to pay $3 billion to settle charges related to several drugs, including Avandia, but the Justice Department has declined to comment on that one as well.
Jones provided The Inquirer with state figures sent to Grassley's office in 2010 by Michael Nardone, then an official with the Pennsylvania Medical Assistance Program.
New Jersey never responded to Grassley's 2010 request for information nor a follow-up letter dated Jan. 24. State officials could not be reached for comment Tuesday. Delaware Medicaid officials responded to Grassley in 2010 and again in February.
J&J's Risperdal lost patent protection at the end of 2007, so the 2008 figures were the beginning of the decline in costs as generic versions were used more often.
As a comparison, AstraZeneca P.L.C.'s antipsychotic, Seroquel, is just now losing patent protection on most versions.
In 2008, the top 10 prescribers in Pennsylvania wrote 18,705 prescriptions for Seroquel, costing Medicaid $3.67 million. State officials provided The Inquirer with the most recent response to Grassley. That letter says the top 10 prescribers wrote 17,692 scripts for Seroquel, costing Medicaid $5.73 million.
In 2010, AstraZeneca paid $520 million to settle charge of illegal marketing of Seroquel.
"I liken the DOJ effort to a storm surge building for a long time," Jones said, crediting Grassley and a few others in Congress for helping to push the issue. "They have a clear eye on the dirty ways of fraudulent marketing and are systematically exposing it. It is changing the way antipsychotic drugs are marketed in America."
Contact David Sell at 215-854-4506 or
dsell@phillynews.com.

The government in India has granted the rights to an indigenous pharmaceutical group to manufacture a generic version of the cancer treatment drug Nexavar.
For the first time, an Indian drugs firm has been approved to produce a medication under licence when the original's still patent-covered.
As a result of the agreement, the firm - Natco Pharma - is obliged to forward six per cent in royalties back to Bayer, which presently markets Nexavar alongside Onyx Pharmaceuticals.
Bayer, meanwhile - according to reports - isn't best pleased with the Indian government's move. "We are disappointed about this decision", company representative Sabrina Cusimano stated in comments made to the Associated Press. "We will see if we can further defend our intellectual property rights in India".
Nexavar Cancer Drug
Nexavar is the market name for sorafenib, an orally-taken medication now approved to treat two types of cancer - advanced hepatocellular carcinoma (liver cancer and advanced renal cell carcinoma (kidney cancer .
The kidney cancer approval came first, in 2005, when the US FDA declared its satisfaction with the product. It did the same for the drug as a liver cancer treatment two years later and, with clinical trials now in progress, thyroid cancer could be the next condition added to this approved treatment list.
Controversially, the drug's not available as a
UK liver cancer treatment, after being rejected - on grounds of cost - by the National Institute for Health and Clinical Excellence in November 2009.
Natco Generic Nexavar Approval
The Natco generic Nexavar approval decision will see the production of drug copies priced at the equivalent of £112 for a box of 120: less than £1.00 each. This is dramatically cheaper than the original drug, with the same quantity presently priced at over 30 times that cost.
The Indian pharmaceutical firm believes that the drug's availability is key to the treatment of close to 9,000 cancer patients in India.
"This is a victory for Indian patients and for India's generic manufacturers, which are under attack", Natco Pharma's General Manager, Madineedi Adinarayana, stated according to the BBC, adding: "many more such cases will follow."
West Yorkshire.
?19,000 - ?24,000
My client is an established and growing pharmaceutical company. They are currently looking for an addition to their Formulation team, reporting to the formulation team leader.
The main responsibility of the role is to develop suitable and stable formulations and processes using formulation aids and processing techniques. To support Analytical Development throughout Formulation Development.
To develop robust and stable formulations for new products that complies with the requirements of marketing.
Multi-project management of new formulations, from initial project approval through to successful production scale up, and licence regulatory submission (where applicable .
Liaise with multiple departments to facilitate new product introduction.
To produce process development protocols and reports to provide evidence of robust product manufacture.
To produce outline methods of manufacture for production.
To ensure that the batch size and methods of manufacture are of sufficient scale necessary to fulfil the marketing requirement, achieved by supervision of batch manufacture (In conjunction with production .
To produce specifications for new raw materials and new products.
To produce reports to support process development and validation activities.
To produce documentation which ensures that stability requirements are met.
To provide support for MA applications, providing information for Development Pharmaceutics reports as directed by Regulatory requirements.
To characterise suspensions with respect to particle size and rheological properties, providing supporting data and reports described above to support MA applications.
To review existing products and processes, recommending improvements, as new techniques become available.
Ensure all work performed complies with GLP, cGMP where applicable.
To keep accurate records of all work undertaken as per internal/external procedures/guidelines.
The successful applicant for this role will hold a BSc. in Pharmacy or a related subject with experience in pharmaceutical formulation. Experience of oral liquid dosage would be desired but not necessary.
If you are interested in applying for this role, then please forward your CV to: Brendan Rogers at PULSE Scientific.
Post : Business Analyst in Pharmaceutical Research
Experience : 0 to 2 yrs
Job Desciption :
a. Track the pharmaceutical, biotech and medical devices industry, especially therapeutic areas/modalities of interest to our regular clients
b. Understand project brief given by clients/project leaders, create analysis plan, data requirements, research approach, and prepare reports or executive presentations with key insights and recommendations
c. Report to project leader/consultant and assist in executing or execute the projects completely depending on complexity and scale
Education :
M.Sc
- Bio-Chemistry,
Biotechnology
,
Life Science
Salary : INR 1,75,000 - 2,50,000 P.A. The CTC is exclusive of Performance Bonus
Desirable :
a. High level of comfort with MS Excel, Power Point, ability to perform basic calculations (basic financial, statistical and data manipulation understand sales and marketing performance metrics, basic data cleaning and organizing, ability to create meaningful charts/ visual representation to simplify complex analyses.
b. Web search, high level of familiarity with life sciences lexicon, regulatory framework for
pharmaceuticals and biotech
c. Attention to detail, accuracy, business relevance in analysis and report writing/executive
presentation skills
Location : Bangalore
Contact Details :
Executive Name : Ravi Prakash
Stratycon Business Solutions Pvt Ltd
Address : 14th cross, 2nd block,
Jayanagar Bangalore,Karnataka,India 560011
Email Address : raviprakash@stratycon.com
Deadline : 09.04.12
A medical doctor and former consultant to Takeda Pharmaceuticals is charging in a whistleblower lawsuit that the company failed to report serious adverse events related to the antidiabetes drug pioglitazone (marketed as Actos .
Helen Ge states in her lawsuit that Takeda “instructed medical reviewers not to report hundreds of non-hospitalized or non-fatal congestive heart failure cases as ‘serious’ adverse events and thus avoided its responsibility of accurately analyzing and reporting these hundreds of serious adverse events to the FDA [US Food and Drug Administration].”
She also claims that the company failed to report 28 of 100 cases of bladder cancers to the FDA, which she called a “serious discrepancy,” and that Takeda told her to change her notation that the cancers were “related” to “unrelated.” The suit says that she was fired after she complained to her supervisors about the company’s failure to report all serious adverse events.
The suit was originally filed in June 2010 but wasn’t publicly disclosed until 24 February, when the federal government declined to join the suit.
Dr Ge charges that Takeda “orchestrated” the downfall of its main competitor drug, rosiglitazone (GlaxoSmithKline’s Avandia , which is in the same class of drugs. In her suit she claims that after the
New England Journal of Medicine
published a study in 2007 (2007;356:2457-71 showing that rosiglitazone increased heart attacks and congestive heart failure, the FDA asked GlaxoSmithKline and Takeda in May 2007 to add warnings about heart failure for both drugs.
According to the suit, in November 2007 Takeda “began to run print advertising in 82 major market newspapers and national publications such as
Time
and
Newsweek
, advising readers with type 2 diabetes that ‘Actos has been shown to lower blood sugar without increasing your risk of a heart attack or stroke.’” At the same time Takeda stopped reporting non-fatal or non-hospitalised heart failure events as “serious,” while GSK continued to report all heart failure events as serious, unfairly positioning pioglitazone as safer than rosiglitazone.
Whether pioglitazone is actually safer than rosiglitazone is controversial. A 2010 meta-analysis by Steven Nissen, chairman of the department of cardiovascular medicine at the Cleveland Clinic in Cleveland, Ohio, reported that when compared with control treatment rosiglitazone increased heart attacks but not the rate of heart failure or cardiovascular mortality (
Archives of Internal Medicine
2010;170:1191-201, doi:
10.1001/archinternmed.2010.207 . David Graham, a safety officer with the FDA, conducted an observational, retrospective, inception cohort study of 227?571 patients aged more than 65 years to compare rosiglitazone and pioglitazone and reported that rosiglitazone was associated with “an increased risk of stroke, heart failure, and all-cause mortality and an increased risk of the composite of AMI [acute myocardial infarction], stroke, heart failure, or all-cause mortality” (
JAMA
2010;304:411-18, doi:
10.1001/jama.2010.920 . However, a retrospective cohort study published in 2010 in
Circulation
that assessed 36?628 patients taking either rosiglitazone or pioglitazone found no differences in overall heart failure, acute myocardial infarction and all cause mortality (2010;3:538-45, doi:
10.1161/CIRCOUTCOMES.109.911461 .
Dr Nissen told the
BMJ
that FDA statisticians “showed unequivocally that pioglitazone is less likely to cause ischaemic events than rosiglitazone.” He said, “These findings were confirmed by the analysis published in
JAMA
” and that “since several of these analyses” did not rely on data reported by Takeda “the differences are likely [to be] real.” He added that both drugs, however, “can precipitate congestive heart failure and cause fractures.”
France suspended pioglitazone in June 2011 over concerns about bladder cancer. Rosiglitazone is available in the United States with severe restrictions (
BMJ
2010;341:c5287, doi:
10.1136/bmj.c5287 , and the European Medicines Agency has suspended its marketing approval (
BMJ
2010;341:c5291, doi:
10.1136/bmj.c5291 .
Takeda said that it can’t comment on the allegations while the lawsuit is pending but that it “complies with all laws and regulations regarding the reporting of adverse events.” Dr Ge declined to comment.
Notes
Cite this as:
BMJ
2012;344:e2002
By Jeanne Lenzer
Salary: 110000
Description: The position requires demonstrated expertise in general mammalian toxicology. The position will oversee the conduct of general toxicity and drug safety studies in accordance with current scientific and applicable regulatory standards (ICH, FDA and GLPs and will monitor safety studies related to drug registration. Preferably, the incumbent will be specialized in one or more of the following areas: general safety evaluation, genetic toxicology, safety pharmacology, reproductive toxicology, carcinogenicity evaluation. In addition, he/she is expected to provide technical leadership in non-clinical drug safety assessment in support of worldwide clinical and preclinical R&D. The incumbent will be required to make both informal and formal presentations to internal staff and project teams and may be asked to assist with presentations to senior management as well as providing project team representation and active involvement in process development. The incumbent will be expected to contribute to the preparation of relevant sections of drug registration submissions. Knowledge of the drug development process and experience in interaction with scientific disciplines such as pharmacokinetics, metabolism, medical research and clinical pharmacology are essential. A successful track record of toxicology testing program management, innovative thinking, execution and timely delivery of reports and regulatory submissions is helpful. PRINCIPAL ACCOUNTABILITIES: Direct responsibilities will include, but are not limited to the following: o Participation in the evaluation and qualification of CROs. o Assists in the design of study protocols for nonclinical toxicology and safety pharmacology studies for conduct at CROs. o Conduct, monitor, and manage toxicology studies at CROs. This will involve limited travel (~10% . o Reviews study data and reviews/revises final study reports for department authorization. o Assists in the preparation of the nonclinical safety sections of regulatory submissions for registration/marketing authorizations of new drug products o Provide technical expertise to support project teams goals and objectives. Educational Requirements A minimum MS in pharmacology, toxicology, biochemistry or related science is required. At least 5 - 7 years industry (preferably pharmaceutical experience with 3 + years' experience in the conduct of mammalian toxicology studies. Required Experience & Technical Requirements o Knowledge and expertise in toxicology and standard and alternative toxicology test methods is necessary to address the range of varied chemical substances that will need to be tested and to interpret and report the study results. o Knowledge of GLPs and direct experience in toxicology testing and/or study management is needed to successfully prepare protocols, implement, and adequately oversee the toxicity studies conducted by CROs and to understand the relative strengths and limitations of testing methods as they relate to hazard identification. o Knowledge and experience in internet/database searching is necessary to develop information that will allow the avoidance of duplicative and unwarranted use of animals in toxicology testing and to assure up-to-date and complete knowledge of available toxicology data and regulatory changes and initiatives. This includes but is not limited to experience in successfully searching FDA, EMEA, WHO, NTP, ASTDR, IARC, NLM databases. o Concise and accurate writing skills and clear and effective oral presentation skills.
Contact:
dld@judge.com
This job and many more are available through The Judge Group. Find us on the web at
www.judge.com
MUMBAI: In a landmark decision that could set a precedent on how life-saving drugs under patents can be made affordable, the government has allowed a domestic company, Natco Pharma, to manufacture a copycat version of Bayer's patented anti-cancer drug, Nexavar, bringing down its price by 97%.
In the first-ever case of compulsory licencing approval, the Indian Patent Office on Monday cleared the application of Hyderabad's
Natco Pharma to sell generic drug Nexavar, used for renal and liver cancer, at Rs 8,880 (around $175 for a 120-capsule pack for a month's therapy.
Bayer offers it for over Rs 2.8 lakh (roughly $5,500 per 120 capsule. The order provides hope for patients who cannot afford these drugs.
The approval paves the way for the launch of Natco's drug in the market, a company official told TOI, adding that it will pay a 6% royalty on net sales every quarter to Bayer. The licence will be valid till such time the drug's patent is valid, i.e. 2020. As per the CL (compulsory licence order, Natco is also committed to donating free supplies of the medicines to 600 patients each year.
Bayer said it was "disappointed" and would "evaluate options to defend intellectual property rights" in the country. In July 2011, Natco had applied for the CL in the Mumbai patent office to make Sorafenib Tosylate for which Bayer has a patent in the country since 2008.
Responsibilities:
-Manage the collection, documentation and communication of all Pharmacovigilance / Drug Safety reports
-To contribute to the approval of promotional documentation produced in support of marketed products in accordance with the ABPI / PAGB Codes of Conduct
-Management of safety and other documentation ensuring that the documents are up to date, scientifically accurate and approved for the use in the UK
-Work closely with commercial department, including medical advisors, scientific advisors, sales and marketing
-Continue to provide up to date medical, technical and Pharmaceutical information on all of the companies' products to both internal and external customers.
-Regularly update all relevant information sources, including databases and factsheet
-Work with Medical Affairs to ensure the best possible outcomes from submissions
-Participate in promotional material screening
Arrow Generics UK has launched generic versions of AstraZeneca’s Zomig (Zolmitriptan and Zomig Rapimelt (Zolmitriptan Orodispersible in the UK and France.
Arrow, part of the Watson Group, released the generics after the patent expired for the two treatments indicated for patients with migraine headache with or without aura.
Last year, the two treatments had sales in the UK and France of around $107 million in the two markets, according to IMS Health data.
The two generics are also set to be launched in the Nordic region later this month on the date of expiry.
Joining Zolmitriptan and Zolmitriptan Orodispersible, Arrow has also launched a generic alternative of GSK’s Naramig (Naratriptan – indicated for the same condition.
It's hard to know these days which way the proverbial worm is turning when it comes to shifts in drug policy. Election years tend to do that. Despite an historical turn of events in Central America which saw Presidents of drug trafficking nations come together to call for world wide decriminalization of drugs, in an effort to end the violence and corruption of the drug trade, the US continues to demur, absurdly claiming that the "War on Drugs" has been a success. Even stranger is Canada's recent announcement that they plan to follow the US model of a "tough on crime" approach to drug policy, which threatens to swell their correctional system in the same ways as in the US. Still, good news abounds with recent studies showing that LSD can cure alcoholism, psychedelics can cure PTSD, and cannabis smoking is not nearly as harmful as the prohibition governments claim. ~ CS
Google+ Presents:
It's Time To End The War On Drugs
To liberalise or prohibit, that is the question. And to answer it the masters of live debate have joined forces with the masters of web technology to create a never-seen-before combination of Oxford debating and Silicon Valley prowess.
Prohibitionists argue that legalising anything increases its consumption. The world has enough of a problem with legal drugs like alcohol and tobacco, so why add to the problem by legalising cannabis, cocaine and heroin?
The liberalisers say prohibition doesn’t work. By declaring certain drugs illegal we haven’t reduced consumption or solved any problem. Instead we’ve created an epidemic of crime, illness, failed states and money laundering.
Julian Assange and Richard Branson; Russell Brand and Misha Glenny; Geoffrey Robertson and Eliot Spitzer. Experts, orators and celebrities who’ve made this their cause – come and see them lock horns in a new Intelligence?/Google+ debate format. Some of our speakers will be on stage in London, others beamed in from Mexico City or Sao Paulo or New Orleans, all thanks to the “Hangout” tool on Google+.
The web will have its say, and so can you at the event in London. Be part of the buzz of the audience, be part of an event beamed across the web to millions. Come and witness the future of the global mind-clash at the first of our Versus debates, live at Kings Place
Source:
Intelligence 2 from Google +
North America
America's plague of incarceration
The message is (or should be deeply disturbing. Shouldn't the USA be ashamed at having the world's largest prison system and highest incarceration rate (754 per 100 000 people ? The richest country in the world has so many of its citizens in prison that it can't afford to house them with even basic minimum medical care (more than half of all prisoners have mental health or drug problems . Prison overcrowding itself has become so terrible in California, that in May, 2011, the US Supreme Court affirmed a lower court order that California release some 46 000 prisoners because of the inhuman conditions under which they were being held. In the Court's words, “A prison that deprives prisoners of basic sustenance, including adequate medical care, is incompatible with the concept of human dignity and has no place in a civilised society.”
Source:
"
A Plague of Prisons: The Epidemiology of Mass Incarceration in America,"
The Lancet.
International Women's Day: U.S. Must Address Impact of Mass Incarceration on Women.
More women are ending up behind bars than ever. Between 1980 and 1989, the number of women in U.S. prisons tripled. And the number of women in prison has continued to rise since. In the last 10 years, the number of women under jurisdiction of state or federal authorities
increased 21 percent to almost 113,000. During the same time period, the increase in the number of men in prison was 6 percentage points lower, at about 15 percent. The increase in women in the federal population was even larger- over 41 percent from 2000 to 2010.
Most women are incarcerated for nonviolent offenses. Over one-fourth are in prison for a drug offense, while 29.6 percent were convicted of a property crime. Addiction plays a large part in a number of women's property crimes, and a lack of available or appropriate treatment only serves to drive their contact with the justice system.
Source:
Justice Policy Institute
From Cell to Screen: The Story of Mumia Abu-Jamal -- Part I

Stephen Vittoria is that rare commodity in Hollywood today: a filmmaker with a conscience. To be more precise, a filmmaker with a strong political conscience. After making two feature films,>Black and White& Hollywood Boulevard (1996 , as well as three feature documentaries:Save Your Life -- The Life and Holistic Times of Dr. Richard Schulze (1998 ,;Keeper of the Flame (2005 and the award-winning art house hit One Bright Shining Moment: The Forgotten Summer of George McGovern (2005 , a portrait of the South Dakota senator who tried to unseat Richard Nixon from the White House in 1972.
For his latest exploration into America's socio-political landscape, Vittoria joins forces with radio producer Noelle Hanrahan to bring Long Distance Revolutionary, the story of Mumia Abu-Jamal, to the screen. Born Wesley Cook in Philadelphia, Abu-Jamal made his name as a tireless writer and journalist during the racially-charged 1970s that often portrayed the City of Brotherly Love as anything but. With his intense coverage of the MOVE organization, a black empowerment group whose ongoing battle with the police and city hall came to a fiery end in 1985, Abu-Jamal become a constant thorn in the side of the city's powerful establishment. Things came to a sudden head for Abu-Jamal himself on the evening of December 9, 1981 when he was accused of murdering a Philadelphia police officer. He received a death sentence the following year, and has been on Pennsylvania's death row until early this year, when his death sentence was commuted to a life sentence in December, 2011.
Abu-Jamal's case remains one of the most controversial and heatedly debated in American legal history, with participants on both sides either protesting his innocence in the murder of Officer Daniel Faulkner or his absolute guilt with equal passion and more often, great vehemence.
Source:
Huffington Post
What’s In a Name? A Lot, When the Name is “Felon”
At a
recent conference of journalists at John Jay College, I raised an issue I have about language in the media: the frequent use of the word “felon” to describe a person who has been convicted of a crime.
“Felon” is an ugly label that confirms the debased status that accompanies conviction. It identifies a person as belonging to a class outside many protections of the law, someone who can be freely discriminated against, someone who exists at the margins of society.
In short, a “felon” is a legal outlaw and social outcast.
Source:
The Crime Report
Addiction: Medical Disease or Moral Defect?
Scientific theories that addiction hijacks the brain have just increased the stigma that they were meant to stop. At least in the moralistic bad old days, addicts were still viewed as having free will. Here's an alternative to both of these no-win approaches.
Source:
The Fix
Scientists Explore Hallucinogen Treatments for PTSD, Sex Abuse Victims
Mind-altering compounds, such as LSD and psilocybin, stirred controversy in the 1960s. As the counter-culture’s psychedelic drugs of choice, the widespread use - and abuse - of hallucinogens prompted tougher anti-drug laws.
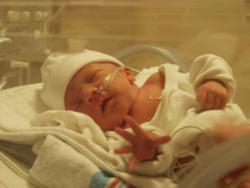
On March 6, 2012, the Food and Drug Administration (FDA approved Surfaxin to treat respiratory distress syndrome (RDS . RDS is a potentially dangerous condition that occurs when an infant is incapable of naturally producing pulmonary surfactant, a liquid coating inside the lungs that helps to keep them open. Premature infants may show signs of RDS at birth or within the first few hours after delivery.
Biopharmaceutical company Discovery Laboratories Inc. develops surfactant technology for surfactant replacement therapies (SRT to treat various respiratory diseases. The company filed for a new drug application in 2004. Since then, Discovery Laboratories has been denied marketing permission four times. In 2005, an “approvable letter” was sent stating that the new drug application will be reviewed and included specific terms and conditions that must be met prior to gaining U.S. marketing permission.
In 2009, the FDA asked Discovery to validate a biological activity test (BAT to ensure Surfaxin’s quality and stability. A number of meetings were scheduled with Discovery to discuss details of the BAT and to establish a plan for moving forward before Surfaxin could be commercially marketed in the United States.
Last month, an Advisory Panel for the FDA recommended that Surfaxin be approved. Surfaxin is the fifth drug to be approved in the U.S. for RDS treatment. It is the first synthetic peptide containing surfactant to be approved for commercial use in neonatal medicine. It is estimated that 90,000 premature infants receive animal deprived surfactant treatments each year in the U.S.
Moving forward
Discovery Laboratories states that commercial marketing of Surfaxin will take place later this year. The company anticipates sales to range between $50 million and $75 million. It also plans to conduct clinical programs for acute respiratory distress syndrome (ARDS in adults, neonatal respiratory disorders (NRD in infants, severe asthma in adults, and other respiratory diseases.

If there is one buzzword of economic policy this year it is productivity.
Want to be a politician, policy wonk or media commentator and you want to deliver or analyse economic policy? Make sure you throw in a good helping of "productivity".
It has quickly become the tomato sauce of policy - put it on top of everything in the belief that it makes everything taste a bit better or at least takes away the flavour of the bad policy underneath.
In the past week Wayne Swan, Tony Abbott, Joe Hockey, Secretary of the Treasury Martin Parkinson, and Reserve Bank Governor Glenn Stevens have all either made speeches mentioning productivity, or - in the case of Stevens - including mention of it in his statement announcing the cash rate.
Actually concern about productivity is no recent phenomenon. It has long been regarded as the key to economic growth and improvement in standard of living. And if there is one rule about discussing productivity it is that at some point you quote Nobel Prize-winning economist Paul Krugman who wrote back in the 1990s:
Productivity isn't everything, but in the long run it is almost everything. A country's ability to improve its standard of living over time depends almost entirely on its ability to raise its output per worker.
So it seems pretty important then. So important that it probably deserves to be treated with a bit more respect than it currently is by many in politics, business and the media - where it is now so widely used that quite often it becomes divorced from its actual meaning and refers to whatever the speaker or writer wants it to mean.
Aside from the great words of Professor Krugman, why is productivity such a concern? The main reason is the graph below. Productivity is generally (and very roughly defined as GDP per hour of work. Given that as a rule the national productivity increases over time, we care less about the actual amount of "GDP per hour worked" and more about the level of growth of GDP per hour worked. As you can see the graph below shows this growth in productivity has been declining since around 2000.

Now if quoting Paul Krugman is the first rule of discussing productivity, the second rule seems to be to ignore that he says "it isn't everything" and to assume he meant "it is everything". The next step is to confuse productivity with labour participation and output.
To explain, take this example:
Imagine if you work at a pie-making factory with five workers that produces 500 pies a day (or 100 per worker, or 12.5 pies per hour . Then one day your boss comes and says the company is hiring two young people and two seniors and that this will improve productivity. If your boss did say that what she/he would in effect be saying is she/he is hiring two young people and two seniors who will be able to produce more pies per hour than what you and the rest of your co-workers can produce. Remember productivity is output per hour worked. So if these new employees actually were slower at their job than you and your co-workers then productivity would actually decline. Let's say these new workers only can produce 10 pies an hour. They combined would make 320 pies a day. The original five workers churn out their usual 500, meaning the company now makes 820 pies a day, but the average pies per worker per hour is now 11.4 pies per hour.
Labour participation has increased (four new workers ; output has increased (320 more pies , but productivity has decreased.
Now is that a bad thing? Not for the four new workers - they have a job, and it may not be bad for the company - especially if can sell the extra 320 pies. But if it wants to make the same profit per pie it will actually have to increase the price it sells them for. So it might end up bad for those who buy the pies, because now they have to pay more for them.
I use this example because last year Tony Abbott announced a productivity policy:
Over the past two years, GDP per hour worked has not grown at all. To improve productivity, the Coalition will provide incentive payments to employers who take young people and seniors off welfare into work.
Now that policy might increase national labour participation, it might increase national output (GDP , but will it "improve productivity"? In the short term, almost certainly not - the new workers will have to be trained and while they are being trained their productivity will be lower than the other workers, thus productivity will decline. In the medium to long term it is also unclear how productivity will increase unless for some reason these new workers are trained better than the current workers and thus become more productive than the current workers.
Joe Hockey committed the same error last week in his speech to the Sydney Business Chamber in which he promised that that the Liberal Party would offer "a souped up productivity agenda". He stated:
The sad truth is that productivity growth has stalled under this Government. GDP per hour worked fell over the 18 months to September quarter 2011 [11]. Australia has been producing less for a given effort.
…
The Coalition has a compelling strategy to help lift productivity.
First will be genuine welfare reform to lift participation in work since there is no-one so unproductive as the person who is able to work but is not doing so.
Well if by a "compelling strategy" he means one that may increase participation but is pretty much guaranteed to reduce productivity in the short term, then yes, it is. Hockey's statement about "there is no-one so unproductive as the person who is able to work but is not doing so" sounds quite reasonable and logical at first blush.
The only problem is it has nothing to do with the problem of productivity that he states in the first part when he talks of "stalled productivity growth".
To go back to the example of the pie factory, Hockey is suggesting productivity has increased because at first there were nine people making 500 pies (but four were unemployed , and after the four new people were hired the nine people were making 820 pies. Huzzah! Productivity increase!
Except we don't measure productivity that way, because we already have a category that measures output per person - it's GDP per capita. We also already have a category to measure the rate of participation in the workforce. It's called, funnily enough, the "participation rate". Here's a look at how it has been doing since 1978 (the same period as the productivity growth graph above :

The rate has been climbing the whole time and exploded in the 1980s from 1983 to 1990, and again from 2004 to 2009. In the 1980s this was due to a big surge in women entering the workforce. In February 1983 only 44.5 per cent of Australian women were either working or looking for work; by 1990 it had increased to 52 per cent. The participation burst from 2003 to 2009 was due to an increase in the participation of both men and women.
But if you look at the participation rate and the productivity rate graphs when participation was increasing in the late 1980s, productivity growth was declining – and a similar thing happened during the 2003-2009 participation burst. There was an increase in both productivity growth and participation in the mid 1990s, though you would be hard pressed to suggest a correlation. The best you can argue is that increased participation increases productivity in the mid-long term.
What it shows is that to increase productivity you need to do more than just get people into the workforce.
A similar error occurs when talking of paid parental leave. The Liberal Party's proposed plan is frequently linked in the media with productivity.
Except when the Productivity Commission looked at Paid Parental Leave back in 2009, the one aspect it was very hedgy on was the gain in productivity. In fact it found that for many companies productivity would decline because during the parental leave a temporary worker would need to be brought in, who would most likely be less productive than the person on leave. One area it suggested there may be productivity increase in the long term is for the children of those parents who are able to take the leave, because of the positive effects of parent-child bonding in those first six months.
When the Productivity Commission talked of productivity benefits of parental leave for companies it mainly discussed for individual companies that offered such leave compared to those who don't - as the companies that do offer it are more likely to attract higher skilled women. But such benefits are largely removed if all companies have to have in place a paid parental leave scheme.
Now this is not to suggest that a parental leave scheme or encouraging young people and older workers on welfare back to work is a bad thing. Far from it - they are actually excellent policy aims, but not because of increases in productivity - but rather because they will increase participation and output. But it goes to the mistake of politicians, business leaders (and often journalists believing that productivity is everything. In the House of Representatives Committee Inquiry into raising the level of productivity growth in the Australian Economy in 2010 the Department of Treasury nicely explained this fallacy:
Some people who are not currently in the labour force, if you brought them into the labour force, may be less productive than the current average worker. So, if you took a strict measure, you could say they may reduce labour productivity through reducing the average. That might be a nice technical point but it would be a pretty silly conclusion. Given that there are a range of disincentives for participation, removing those and improving overall workforce participation outcomes clearly enhances wellbeing overall.
Thus productivity is not everything - wellbeing is. We want people to come into the workforce (and stay - in the case of parents because young people who get into the workforce are more likely to have a higher lifetime income than those who stay on welfare longer into their 20s, the same goes for families where both parents are able to work. Policies that increase participation and output are worthy enough goals by themselves and certainly do not need to be justified on productivity terms.
Paul Krugman himself noted the problems of being concerned too greatly with productivity when he wrote last year of Ireland, which had apparently seen an increase in competitiveness since its "austerity measures" were introduced after the GFC. He noted:
… what has happened in the austerity era is that sectors, which aren't selling to the domestic market, have held up much better than labour-intensive sectors serving that domestic market. And this causes a spurious increase in labour productivity: if you lay off a construction worker but don't lay off a pharmaceutical worker who basically watches over very expensive machines that produce a lot of output, it looks as if productivity has gone up, but in any individual sector nothing has happened.
This is what happens when we get into trouble if governments and businesses focus purely on productivity and forget that there is no point increasing productivity if it means less people are working.
The other rule of talking about productivity is that it must involve Industrial Relations - and usually assertions that unions are killing productivity. For example BHP Chairman Don Argus in a speech last month stated:
I believe we have seen a major warning light for productivity in the disruption many businesses face from strikes and the rising power of unions.
We see this link often mentioned - especially in The Australian and the Australian Financial Review, which both push pro "flexible labour market" policies.
Last week the Australian Bureau of Statistic released its quarterly " Industrial Disputes" figures. It showed that while work days lost due to strikes had declined in the last quarter, they had risen in the 2011 compared to 2010. The Australian reported it as:
The number of days lost to strikes and lockouts has almost doubled in the past 12 months, in a further brake on the nation's productivity.
What the article didn't say was that while the number of days lost had almost doubled in the past 12 months, so too had productivity:

In 2010, productivity actually declined but number of hours lost due to strikes was also down. In 2011 both productivity and the number of working days lost increased.
This is actually not a unique occurrence. A comparison of productivity and working days lost over the past 20 years shows the same thing:

In the past 20 years the days lost to strikes has dramatically decreased - from an average of 164 days lost per quarter from 1991-2001 to 55 days lost per quarter from 2001-2011. And yet in the 1991-2001 period, the average quarterly productivity growth was 0.6 per cent, compared to 0.2 per cent in the past 10 years.
I don't mean to suggest more strikes leads to more productivity, but to show that linking strikes to declines in productivity is easily done unless you want to find some actual proof, because the data does not show that more strikes leads to a "brake" on productivity. What they may lead to is less output, but that is a very different aspect to productivity. Thus declining productivity growth may be a tad more complex problem than simplistically being the fault of unions or IR policy.
If we are going to talk about productivity (and Australia's problems with it the best thing politicians, business leaders and the media can do is not to suggest productivity is everything, nor to suggest everything should be done or should not be done because of productivity. Similarly realising that productivity is not participation nor output might also help voters so that when we next hear politicians brag about how they will increase productivity we will know if they are producing good policy, or are just increasing the output of bulldust.
Greg Jericho is an amateur blogger who spends too much of his spare time writing about politics. His blog can be found here. View his full profile here.
Johnson & Johnson (JNJ faces a U.S. government demand to raise its offer by $800 million from an initial proposal to settle a federal civil investigation into marketing of the antipsychotic Risperdal, according to three people familiar with the matter.
The Justice Department is demanding that J&J pay about $1.8 billion to resolve the civil claims by the U.S. and some states, the people said. The company raised its offer to settle the civil investigation to $1.3 billion by March 8, and negotiations on a final amount are continuing, one person said.
It is absolutely unacceptable that this industry pushes people to believe that medicine is expensive because research and development is costly. These companies sweep negative clinical trial findings under the rug, invest in developing drugs that may be less effective and more dangerous than ones already on the market and care more about improving a patient’s self-esteem than saving lives.
By Rebecca McGoldrick
Key responsibilities will include:
·Perform safety screening and risk evaluations.
·Development of study protocols and data analysis plans.
·Participates in the development of International strategies and tactics.
·Participate in / chair the Pharmacovigilance working groups and drug safety review teams.
·Interact with MDs / Scientists from Medical, Biostatistian and Regulatory Groups.
·Ensure that all promotional activities comply with the medical and legal requirements and act as legal signatory for local promotional material
·Provide medical and Clinical Review and Expert Report Writing for Marketed Material
Salary: ?50 - 75K (depending on experience
No comments:
Post a Comment